Home / Categories / COMBIFLAM TAB.
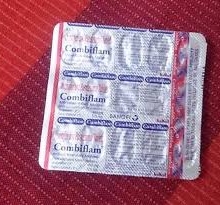
COMBIFLAM TAB.
(15X40)
IBUPROFEN-400MG
PARACETAMOL-325MG
NSAIDS-ANTIPYRETIC/ANALGESICS
AVENTIS PHARMA LTD
Product Details
Ibuprofen
A to Z Drug Facts
Ibuprofen
Action
Indications
Contraindications
Route/Dosage
Interactions
Lab Test Interferences
Adverse Reactions
PrecautionsPatient Care Considerations
Administration/Storage
Assessment/Interventions
Patient/Family Education
(eye-BYOO-pro-fen)Advil, Advil Liqui-Gels, Advil Migraine, Children's Advil, Children's Motrin, Genpril, Haltran, Infant's Motrin, Junior Strength Advil, Junior Strength Motrin, Menadol, Midol Maximum Strength Cramp Formula, Motrin, Motrin IB, Motrin Migraine Pain, Nuprin, PediaCare Fever, Pediatric Advil Drops,  Actiprofen, Alti-Ibuprofen, Apo-Ibuprofen, Novo-Profen, Nu-IbuprofenClass: Analgesic/NSAID
Actiprofen, Alti-Ibuprofen, Apo-Ibuprofen, Novo-Profen, Nu-IbuprofenClass: Analgesic/NSAID
 Action Decreases inflammation, pain, and fever, probably through inhibition of cyclooxygenase activity and prostaglandin synthesis.
Action Decreases inflammation, pain, and fever, probably through inhibition of cyclooxygenase activity and prostaglandin synthesis.
 Indications Relief of symptoms of rheumatoid arthritis, osteoarthritis, mild-to-moderate pain, primary dysmenorrhea, reduction of fever. Unlabeled use(s): Symptomatic treatment of juvenile rheumatoid arthritis, sunburn, resistant acne vulgaris.
Indications Relief of symptoms of rheumatoid arthritis, osteoarthritis, mild-to-moderate pain, primary dysmenorrhea, reduction of fever. Unlabeled use(s): Symptomatic treatment of juvenile rheumatoid arthritis, sunburn, resistant acne vulgaris.
 Contraindications Hypersensitivity to aspirin, iodides, or any other NSAID.
Contraindications Hypersensitivity to aspirin, iodides, or any other NSAID.
 Route/Dosage
Route/Dosage
Rheumatoid Arthritis and Osteoarthritis
Adults: PO 300 to 800 mg tid to qid, not to exceed 3.2 g/day.
Mild-to-Moderate Pain
ADULTS: PO 400 mg q 4 to 6 hr prn.
Primary Dysmenorrhea
ADULTS: PO 400 mg q 4 hr prn.
Juvenile Arthritis
CHILDREN: PO 30 to 40 mg/kg/day in 3 to 4 divided doses.
Fever Reduction
CHILDREN 1 to 12 yr: £ 39.2° C (102.5° F) recommended dose PO 5 mg/kg; > 39.2° C (102.5° F) recommended dose PO 10 mg/kg; maximum daily dose 40 mg/kg.
OTC Use (Minor Aches/Pains, Dysmenorrhea, Fever Reduction)
PO 200 mg q 4 to 6 hr. Do not exceed 1.2 g in 24 hr or take for pain for > 10 days or for fever for > 3 days, unless directed by physician. Use smallest effective dose.
 Interactions
Interactions
Beta-blockers: Antihypertensive effect may be decreased. Digoxin: Ibuprofen may increase digoxin serum levels. Lithium: May increase lithium levels. Loop diuretics: Diuretic effects may be decreased. Methotrexate: May increase methotrexate levels. Warfarin: May increase risk of gastric erosion and bleeding.
 Lab Test Interferences None well documented.
Lab Test Interferences None well documented.
 Adverse Reactions
Adverse Reactions
CV: Peripheral edema; water retention; worsening or precipitation of CHF. CNS: Dizziness; lightheadedness; drowsiness; vertigo; headaches; aseptic meningitis. EENT: Visual disturbances; photophobia; tinnitus. GI: Gastric distress; occult blood loss; diarrhea; vomiting; nausea; heartburn; dyspepsia; anorexia; constipation; abdominal distress/cramps/pain; flatulence; indigestion; GI tract fullness. GU: Menometrorrhagia; hematuria; cystitis; acute renal insufficiency; interstitial nephritis; hyperkalemia; hyponatremia; renal papillary necrosis. DERM: Rash; pruritus; erythema. OTHER: Muscle cramps.
 Precautions
Precautions
Pregnancy: Pregnancy category undetermined. Lactation: Undetermined. Children: Safety and efficacy not established. Elderly: Increased risk of adverse reactions. GI effects: Serious GI toxicity (eg, bleeding, ulceration, perforation) can occur at any time, with or without warning symptoms. Renal effects: Increased risk of dysfunction in patients with preexisting renal disease.
PATIENT CARE CONSIDERATIONS
 Administration/Storage
Administration/Storage
- Give medication soon after meals or with food, milk, or antacids to minimize GI irritation.
 Assessment/Interventions
Assessment/Interventions
- Obtain complete patient history, including drug history and any known allergies.
- Notify physician if visual changes or indications of GI distress or liver or renal impairment occur.
- Monitor patient's cardiac status: BP, pulse (quality and rhythm), edema, tachycardia, palpitations.
- Assess renal function before and during therapy. Serum creatinine, creatinine clearance, and BUN should be monitored in patients with renal impairment.
- Document any changes in liver function (AST, ALT), eye examinations and Hgb and Hct in patients on long-term therapy.
- Notify physician if indigestion, epigastric pain, unusual bleeding or bruising, or dark tarry stools occur.
OVERDOSAGE: SIGNS & SYMPTOMS Drowsiness, lethargy, GI irritation/bleeding, nausea, vomiting, tinnitus, sweating, acute renal failure, epigastric pain, metabolic acidosis
 Patient/Family Education
Patient/Family Education
- Tell patient to take medication soon after meals or with food, milk, or antacids.
- Tell patient to avoid alcohol and medications containing aspirin, such as cold remedies.
- Advise patient to discontinue drug and notify physician if any of the following occur: Persistent GI upset or headache, skin rash, itching, visual disturbances, black stools, weight gain or edema, changes in urine pattern, joint pain, fever, blood in urine.
- Instruct patient not to take otc preparation for > 3 days for fever and > 10 days for pain and to notify physician if condition does not improve.
- Advise patient that drug may cause drowsiness and to use caution while driving or performing other tasks requiring mental alertness.
Books@Ovid
Copyright © 2003 Facts and Comparisons
David S. Tatro
A to Z Drug Facts